Sulphur Dye : Uses, Properties, Chemistry, Classification and Mechanism
Sulphur Dyes
Sulphur Dyes are a type of vat dye used for dyeing cellulosic fibres. The insoluble pigment is converted into leuco compound by reduction with sodium sulphide and the leuco form is subsequently oxidised inside the fibre. Sulphur dyes are manufactured by heating organic materials with sodium polysulphide in aqueous or alcoholic solution. A variety of organic compounds are used including amines, nitro- compounds, phenols and their derivatives. These dyes almost always contain loosely bound sulphur and liberate hydrogen sulphide when treated with acidic solutions of reducing agents such as stannous chloride. Sulphur dyes are mostly used for cellulosic materials or blend of cellulosic fibers with synthetic fibers such as acrylic fibers, polyamide, and polyester.
History of Sulphur Dyes
In 1873- Cachou de Laval (sawdust, caustic soda and sulphur. In 1893 Vidal Black (fusing para-phenylene diamine with Na2S and sulphur). In 1897 immortal black FF (2,4 dinitro 4 di hydroxy diphenylamine with Na Poly sulphide). In 1896 Red Holliday, (grey, brown and black sulphur dyes by the action of sulphur, alkalic sulphides and many organic compounds.
Sulphur Dye Manufacturing Method
1. Reduction: The dye is taken in an aluminium vessel and mixed well with alkali and added specific amount of water. Particular amount of soda ash is added to neutralize any acid formed in the dyestuff during storage. The quantity of reducing agent depends upon the shape depth and M: L of the bath
2. Dyeing: The dye is kept ready with a small quantity of the alkali stable and compatible wetting agent, a dye bath stabilizer, sodium sulphide and caustic soda. The dye is then added to the solution after 15-25 minutes. The temp is raised to above 180 C and kept for enough time
3. Oxidation: The oxidation is done to reconvert the leuco compound back to insoluble dys
Sulphur Dyes Trade Name
Name Company Country
Thional I.C.I UK
Pyrogene Ciba Switzerland
Sulphonol James Ribison UK
Alcogene American Cyanide USA
Eclipse Geigy
Imperial BASF
Fastness Properties of Sulphur Dyes
- They contain sulphur linkage within their molecules
- Sulphur dyes are insoluble in water
- They are soluble in a solution of sodium sulphide (Na2S)
- The sodium sulphide acts as a reducing agent, severing the sulphur linkage, and breaking down the molecules into simple compounds, which are insoluble in water
- The wash fastness is good and the light fastness is satisfactory
- Have poor fastness to chlorine
Application of Sulphur Dyes:
- The use of sulphur dyes is restricted to dull brown, khaki and navy shades, where a good wash but not boil fastness is required.
- Mostly khaki & navy overalls are dyes with sulphur dyes
- An outstanding member of the family is sulphur black
Features of Sulphur Dyes
- Water Insoluble
- Good Light Fastness with a rating of 4 and 8 on the blue scale
- Excellent wash fastness with rating of 3-4 out of 5 on the greyscale
- Good Chemical Resistance
- Not applicable for protein fibres
Chemical Nature of Sulphur Dye
Sulphur dyes are a type of vat dye used for dyeing cellulosic fibres. The insoluble pigment is converted into the substantive leuco compound by reduction with sodium sulphide and the leuco form is subsequently oxidised inside the fibre. Sulphur dyes are manufactured by heating organic materials with sodium Polysulphide in aqueous or alcoholic solution, or by baking with sodium sulphide and sulphur. A variety of organic compounds are used including amines, nitro compounds, and phenols and their derivatives. These dyes almost always contain loosely bound sulphur and liberate hydrogen sulphide when treated with acidic solutions of reducing agents such as stannous chloride.
The chemistry of sulphur dyes is very complex and little is known of the molecular structures of the dyestuffs produced. The Colour Index only gives the structures of the starting materials that are used to produce these dyes and even then the information can be quite misleading. Two products manufactured from the same starting materials are unlikely to have similar properties even though they may have the same CI number. The actual dyestuffs produced from the same materials by different processes may have quite different dye contents, dyeing characteristics and environmental impacts. Because of the large amounts of sodium sulphide used in the manufacture and application of these dyes, much of which will eventually be found in the effluent, they pose a significant environmental problem. In this respect, sulphur dyestuffs produced in developed Western nations are much more environmentally friendly.
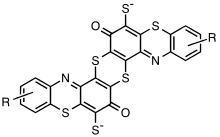
A variety of structural units have been proposed for various sulphur dyes. It is generally accepted that these dyes are polymeric having sulphur-containing aromatic heterocyclic units such as thiazines and thiazoles linked by di- or polysulphide bonds (Figure 17.8). On treatment of an aqueous dispersion of the insoluble pigment with sodium sulphide, the sulphide links are reduced forming individual heteroaromatic units with thiol groups (Scheme 17.4). These are soluble in the alkaline solution in the form of thiolate ions that have low to medium substantivity for cellulose.
Chemistry of Dyes with Sulphur Dye
- The insoluble dye is converted to smaller soluble components called this by reducing agent
(insoluble sulphur dye) (reducing agent) (This)
- These this containing the SH group are readily oxidized in the fibre to the original insoluble sulphur dye, giving a colour with a very good washing fastness
Sulphur Dye Chemical Structure
The exact chemical structure has yet to be discovered. A portion of the sulphur dye molecule is given below
Sulphur Dyes Classification
Sulphur dyes can be classified in many ways. But according to their solubility, there are essentially three classes
- Conventional or water-insoluble dyes: which have no substantivity to cellulosic
- Leuco sulphur dyes: which are water soluble and non-substantive to cellulosics
- Solubillised sulphur dyes: which are substantive to cellulosic
Reducing Steps of Sulphur Dyes
- Break sulphur linkage within the molecules and turn water-insoluble dye into water-soluble leuco form
- Dye-S-S-Dye + 2 [H] = Dye - SH + HS- Dye
Sulphur Dye
- Reducing agents Na2S, Na2S2O4, C2H4O2S
Dyeing of Fbers with Sulphur Dyes
- Dye fabric in the presence of electrolyte
- Soluble sulphur dye + Cotton = Dyed cotton
Oxidation Steps of Sulphur Dyes
- These groups are readily oxidized by the action of atmospheric O2 or any other oxidizing agents and reconvert water soluble leuco form of sulphur dye into the previous water-insoluble form
- Dye - SH + HS - Dye + [O] = Dye-S-S-Dye + H2O
- Oxidizing Agent: K2Cr2O7, Na BO3nH2O, Na2CO3.5H2O2, Na2O2, H2O2
Typical Recipe of Dyeing
1. Reduction
Sequestering Agent - 1cc/L
Dyes - 1% (o.w.f)
Hydrose - 3 gm/L
M:L - 1:70
NaOH - 3gm.L
Ph - 11
Temp - 60 C
TIme - 10min
2. Dyeing
Salt - 15gm/L
Time - 20 minute
Temp - 80 C
3. Oxidation
Sequestering Agent - 1cc/L
Acetic Acid - 2cc/L
H2O2- 3cc/L
pH- (4.5-5.5)
Temp - 80 C
Time - 10min
M:L - 1:60
Dye Solution Preparation
Suppose,
Fabric weight = 3.65gm
Weight of Water = 3.65*70 = 260 mL
Hydrose = 3*260 = 0.78 gm
Sequestering Agent = 260/1000 = 0.26cc/L
Dyes = 0.01* 3.65 = 0.037 gm
Dyeing:
Salt = 15 *0.26 = 3.9gm/L
Oxidation
Sequestering Agent = 3.65*60 (Liquor ratio) = 0.219gm/L
Acetic acid = 2*0.219 =0.438
H2O2 = 3* 0.219 = 0.657cc/L
After Treatment of Sulphur Dye
The washing and light fastness can be improved by using one of the following after-treatment
- Treatment with metallic salt- e.g dicromate and copper sulphate either alone or in combination
- Topping with basic dye - Cotton goods dyed with sulphur can be topped with basic dyes so as to get enhanced brilliant shade and improved washing fastness properties as well
Precautions
- Before evaluating the amount of used chemicals, the weight measuring machine value must remain zero
- Overall dyeing process should be done in an aluminium vessel
- Amount of used chemicals should be counted properly
- Overall dyeing process above the heater must be around given parameter
Stripping of Sulphur Dyes
Treatment with a warm solution of Na2S in the presence of polyvinyl pyrolodine or NaOCl or bleaching powder (2-3 gm/liter of available chlorine) or KMNO4 or NaOCl in presence of NaOH
Defects of Sulphur Dyes
1. Bronziness or Dullness of Shades:
Causes:
- Excessive delay between lifting of the material from the dye bath and washing-off
- Exposure of goods to air while dyeing
- Too much common salts as exhausting agents
- Insufficient Na2S in dyebath
- Strong dye liquor in the dyebath
Remedies:
- Good washing and dilute solution of Na2S (0.1) % at 30 C
- Treatment with boiling soap solution or a strong Na2S solution
- Treatment with a solution containing 10 % saponified palm at 60 C
2. Sulphur Black Tendering:
Causes:
- Gradual oxidation of sulphur to H2SO4 on storage
- After treatment with copper salts
- Presence of Iron an an impurity
- The method of oxidation in uncontrolled manner
Remedies:
- Treatment of dyed material with 1-3 % of K2Cr2O7 and 1-3 % CH3COOH at 60 C temperature for 30 minutes followed by through rinsing
- Treatment with a little CH3COOH
- Treatment with 5gm/liter soda ash after dyeing without rinsing
Uses of Sulphur Dyes
- Umbrella cloth
- Cotton fabric
- Denim fabric
- Rubber material
Advantages of Sulphur Dyes
Foam Dyeing:
Zero Sulphur Product:
- Less toxic product
- Safe for health
- Can be used to dye fabric for upper body products
- Product savings
- Less chemicals require
- Unsurpassed chemical compatibility
- Better fastness properties
Foam Dyeing:
- Getting more attention worldwide
- Eco-friendly
- 99 % water saving
- 89 % less chemical
- 65 % less energy
Disadvantages of Sulphur Dyes
- Inadequate pictorial ability limits the production of light shades
- Limited hue- range true rate, orange and yellow cannot be produced
- Shade lack brightness
- Imrproped by topping with basic dyes
- Bronzing due to heavy dyeing
- Tendering of cotton on stage in a humid atmosphere
- Not applicable on swimming costume for fear of discoloration
- Not applicable to protein fiber due to the high alkalinity of the bath
Difference between Sulphur Dyes and Vat Dyes
1. The vat dye have high color fastness which is uncommon in other dye classes, sulphur dye have good wet fastness
2. Vat dye have poor rubbing fastness, sulphur dye easy to wash
3. Inko dye is a type of vat dye that uses light rather than oxygen to fix the dye, sulphur dye have fair to good light fastness
4. Mostly used in garments industry
5. Vat dyes have limited shade range
6. Vat dye sensitive to abrasion, sulphur dye have good rubbing fastness
7. Complicated application procedure
8. Vat dyes are time-consuming, sulphur dye low cost
9. Slow process, comparatively less time required
10. Not more suitable for wool
Why is Sulphur Dye Called?
- Sulphur dyes are so called because sulphur dyes contain disulphide (S-S) linkage in their chemical structure
- Sulphur black is the most important class of sulphur dyes
- Commercially successful black sulphur was first prepared in 1893
- Applicable to cellulosic materials, not to wool and protein fiber due to strong alkaline condition
Conclusion
Sulphur dye is one of the most important dye in our everyday dyeing application especially an essential dye for our denim industry. Also newer innovation is also going on about this dye and hopefully will be able to utilize this dye without less environment pollution in future
F.A.Q
o
oo
What is an example of a Sulphur Dye?
Example of Sulphur Dyes are indophenol and sulphur black (Cl 5318)
What are Sulphur Dye Used for?
Sulphur dye are mainly used for dyeing of cellulosic materials and cellulosic fibers with synthetic fiber. They can also be used for a limited number of silk and paper applications




Comments
Post a Comment