Study On Dyeing of Cotton Fabric with Reactive Dyes
Dyeing of Cotton Fabric with Reactive Dyes
What are Reactive Dyes?
A dye, that is capable of reacting chemically with a substrate to form a covalent dye substrate linkage, is known as reactive dye.
Here the dye contains a reactive group and this reactive group makes a covalent bond with the fiber polymer and acts as an integral part of the fiber. This covalent formed between the dye molecules and the terminal of cellulosic fiber between the dye molecules and the terminal -NH group of polyamide on wool fibers
History of Reactive Dyes:
On a 100-year celebration of synthetic dye manufacturing, two chemists of ICI company Steppen and Ratte, tried to manufacture new dyestuffs. In 1956, they succeeded in inventing reactive dyes. This was invented for cellulosic fibers.
The first three reactive dyes were Procion Yellow R, Procion Brilliant Red 2B, and Procion Blue 3G. In 1960, they were awarded a gold medal by the Society of Dyes and Colorists. In 1965, These dyes came to our country and became popular in 1980.
Uses of Reactive Dyes:
- In high light and wash fastness requirements, reactive dyes are used
- In batik work, cold brand reactive dye is used
- Mainly used to dye cotton, linen, and viscose rayon. Sometimes can be used for dyeing wool
Trade Name of Reactive Dyes:
- Bezactive (Bezema, Switzerland)
- Ciba (Ciba, Switzerland)
- Dychufix (Hubei Hwalle dyestuff Ind. co)
- Kemafix (Jaychem, India)
- Levafix (Dystar, Germany)
- Procion (Dystar, Germany)
- Jackazol (India)
- Kemazol (Jaychem, India)
- Remazol (Dyestar, Germany)
- Solazol (Solarfine, Taiwan)
- Solacion (Solafine, Taiwan)
Properties of Reactive Dyes:
- Anionic Dye
- Water soluble
- Easy to apply
- Need alkaline condition
- Light fastness and wash fastness are high
- Wide ranges of shades
- Cheap
- Form a strong covalent bond with cellulosic fibers
- Found in liquid, powder, and paste form
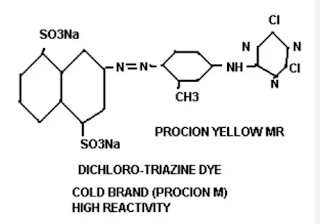
Chemical Structure of Reactive Dye:
The chemical structure of reactive dyes is shown as follows:
Depending on the chemical constitution, there are three types of reactive dyes which are as follows
- Monofunctional Reactive System: Here, only one reactive group
- Bi-functional Reactive System: Here, two reactive groups. Homo-bifunctional reactive system and Hetero bifunctional reactive system
- Poly-functional Reactive System: More than two reactive systems
Dye depending on chemical structure, reactive dyes can be classified as,
- Chlorotriazine Dyes (MCT)
- Vinyl Sulphone Dyes (VS)
- Heterocyclic Halogen Containing Dyes (HHC)
- Mixed Dyes (MCT-VS)
Depending on the application method of temperature, reactive dyes can be classified as,
- Cold Brand Reactive Dyes: This type of applied at very low temperatures such as 25-50 C. They are highly reactive with the fiber
- Medium Brand Reactive Dyes: This type of dye is applied at medium temperatures such as 40-50 C. They have medium reactivity with the fiber
- Low Brand Reactive Dyes: They are applied at high temperatures due to low reactivity to the fiber. temperature such as 60-90 C
Dyeing Mechanism of Reactive Dye:
There are three steps of the dyeing mechanism of reactive dyes which are described as follows:
- Dye absorption: When fiber is immersed in dye liquor, an electrolyte is added to assist the exhaustion of dye. Here, NaCl is used as the electrolyte to accelerate the rate of dyeing. This electrolyte neutralizes the negative charge formed on the fiber surface. So, when the substrates is introduced with the dye liquor the dye is exhausted onto the fiber.
- Fixation: Fixation of dye means the reaction of reactive group dye with terminal -OH or -NH2 of the fiber. Thus forming a covalent bond with the fiber. This is an important phase, which is controlled by maintaining pH by adding alkali. Here, generally, caustic soda, soda ash, or NaHCO3 are used as an alkali depending upon the reactivity of the dye. They create proper pH in dyebath and act as a dye fixing agent. The reaction takes place is shown below:
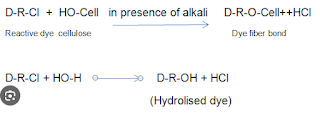 |
| Fixation Phase of Reactive Dye |
3. Washing-Off: As dyeing is completed, a good wash must be applied to the material to remove extra and unfixed dye from the fabric surface. This is necessary for level dyeing and good wash fastness.
Application Method of Reactive Dyes:
- Discontinuous Method:
- Conventional Method
- Exhaust or constant temperature method
- High Temperate Method
- Hot Critical Method
- Continuous Method:
- Pad Steam Method
- Pad Dry Method
- Pad Thermofix Method
- Semi-continuous Method
- Pad Roll Method
- Pad Jig Method
- Pad Batch Method
Stripping of Reactive Dye:
The reactive dye cannot be satisfactorily stripped from the fiber due to the strong covalent bond between the dye molecule and fiber. Stripping becomes necessary when uneven dyeing occurs
Partial stripping: Partial stripping is obtained by treating the dyed fabric with dilute acetic acid or formic acid. Here temperature is raised to 70-10C and treatment is continued until the shade is removed by the desired amount.
Acetic acid -0.5-10 g/L
Temperature 70-100 C
Full stripping: For complete stripping the goods are first treated with hydrosulfite (Hydrose) at a boil and then washed off and bleached with Igl, sodium hypochlorite (NaOCI), or bleaching powder
at room temperature. This is carried out as following steps-
Wetting agent 0.5-1.0gL
NOH 3-6 g/L
Hydrose7-10g/L
Then,
Wetting agent 1g/L (Room Temp x 10min)
Bleaching powder1gt.
(Temp100-105 x 60-30min)
Objectives of Dyeing-
1. To provide color.
2. To obliterate the surface.
3. To develop newer designs as per the market requirement
4. To make it dynamic the following process
5. To improve the weathering properties and durability
Reactive Dye Recipe
Sequestering Agent - 1cc/L
Shade-1%/3% (o.w.f)
NaOH -3gm/L
Na2CO3 - 5gm/L
Salt- 15g/L / 20g/L
PH-11
Temperature - 60°c.
Time- 30 minutes.
Functions of Used Chemicals;
Sequestering Agent- It reacts with metal ions (Ca++, Mg++, Fe++) and removes the hardness of water.
Shade- Modifies the color by decreasing its luminosity by the amount specified in the parameter.
Sodium hydroxide- It converts the oil into water-soluble fatty acid and soap.
Sodium carbonate - It is used to hold the pH in 11.
Salt- It acts as a catalyst that accelerates the dyeing action.
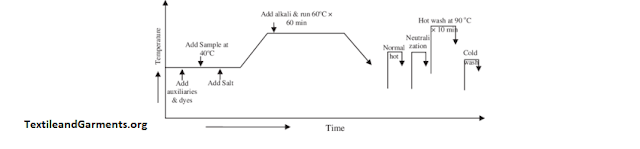
Working Process of Reactive Dye
After measuring the weight of the fabric, we take dye-ligour water. the mixture which is sixty times greater than the fabric weight where the amount of dye labor is added which is calculated by the fabric weight, shade & concentration of the stock solution. We take the overall dyeing process into an aluminium where the fabric is emerged. Then we take a particular amount of Sequestering agent, Sodium carbonate, and 1/3 of actual salt into the mixer. Then, we added half the amount of alkali and consequently two times after 5 & 10 minutes respectively, where the temperature of the mixture was 60 C. After 15 minutes. fabric is taken away for the washing and dyeing successively. Then we measured the material. the weight of the dyed fabric
Fabric weight - 6.24 gm
Dye liquor water = 60*6.24g = 374.4 gm
Amount of liquor water = Fabric weight * Shade %/ Concentration of Stock Solution
= 6.24 *1 / 0.5 = 12.264gm
Weight of Water = (374.4 - 12.264) = 362.2 gm
Sequestering Agent = 374.4/1000
NaOH = 3 * 374.4 /1000 = 1.1232gm
Sodium Carbonate = 5* 374.4 / 1000 = 1.872gm
Salt = 15 * 374.4/ 1000 =5.62gm
Weight after dyeing = 5.94 gm
Precautions:
- Before evaluating the amount of used chemicals, weight measuring m/c must remain zero
- Overall dyeing action should be done in an aluminum vessel
- The amount of used chemicals should be counted consciously.
- Overall, the dyeing process above the heater must be around 60
Conclusion
Dyeing is a very important process for producing garment products. From this experiment, we can learn how to dye the bleached fabric with reactive dyes




Comments
Post a Comment