Textile Dyeing Process Flow Chart
Textile Dyeing Process Flow Chart
Dyeing Process
Dyeing is the process of applying colors to a textile material in loose fiber, yarn, cloth, or garment form by treatment with a dye. It is also the coloring process of textile material by immersing them in an aqueous solution of dye, called dye liquor. Normally the dye liquor consists of the dye, liquor, and the auxiliary. To increase the dyeing effectiveness, heat is applied to the dye liquor.
Woven Fabric Dyeing Process Flow Chart
- Grey Fabric
- Singeing
- Desizing
- Scouring
- Bleaching
- Mercerization
- Dyeing
- Washing/ Drying
- Heat Setting
- Inspection
- Printing
- Steaming
- Washing
- Drying
- Heat Setting
- Finishing
- Finished Fabric
Knit Fabric Dyeing Process Flow Chart
- Grey Fabric Inspection
- Batching
- Fabric turning
- Loading to the machine
- Pre-treatment (Scouring and bleaching)
- Dyeing
- Dewatering
- Drying
- Compacting & Calendering
- Final Inspection & Packing
Hard Water
The hardness of Water can be defined as the presence of soluble salt of Ca and Mg ions in water. The sodium salt of Aluminum and Manganese can also contribute to the hardness of water/
Hardness can be defined as
- Temporary
- Permanent
Consequence of Using Hard Water
- Precipitation of Soap
- Redeposition of dirt and insoluble soap
- Precipitation of some dyes
- Scale formation on equipment and boiler
- Reduction of the activity of enzymes
- Decreases solubility of the sizing agent
- Coagulation of some types of point paste
- Incompatibility with chemicals in finishing
PPM
PPM means parts per million. PPM means there is no 1 kg hardness in 1 million liters of water in terms of CaCO3. It is also measured as mg/l.
106 contains = 1kg hardness
= 1000gm hardness
= 1000*1000 mg hardness
= 106 mg hardness
100000 litre of water contains = 1000000 mg of hardness
1 liter contains = 1 mg of hardness
(0-60) PPM = Soft Water
(60-120) PPM = Moderate Water
(120-180) PPM = Hard Water
(180-350) PPM = Very Hard water
Softening of Water:
The term softening is applied to the process by which we remove or reduce the hardness of water whether it's temporary or permanent.
Temporary Process
1) Boiling of water: Ca(HCO3)2 = CaCO3 + CO2 + H2O
Mg(HCO3)2 = MgCO3 + CO2 + H2O
MgCO3 + H2O = Mg(OH)2 + H2O + CO2
2) Lime Process: Ca(HCO3)2 + Ca(OH)2 = CaCO3 + H2O
Mg(HCO3)2 + Ca(OH)2 = MgCO3 + Ca(OH)2 = Mg(OH)2 + CaCO3
Temporary & Permanent Process
3) Soda Lime Process:
CaCl2 + Na2CO3 = CaCO3 + NaCl
CaSO4 + Na2CO3 = CaCO3 + Na2SO4
MgCl2 + Ca(OH)2 + Na2CO3 = Mg(OH)2 + CaCO3 + NaCl
MgSO4 + Ca(OH)2 + Na2CO3 = Mg(OH)2 + CaCO3 + Na2SO4
Ca(HCO3)2 + Ca(OH)2 = CaCO3 + H2O
Mg(HCO3)2 + Ca(OH)2 = MgCO3 + Ca(OH)2 = Mg(OH)2 + CaCO3
4) Softening of Ion Exchange Material:
Inorganic
- Zeolite [Na2O.Al2O3.SiO2.H2O] Natural
- Permutit [Obtained from fusing together of sodium carbonate, alumina & silica] Manufactured
Both permit and zeolite are soluble in water and can be represented as Na2Z
Na2Z + Ca++ = CaZ + 2Na+
Na2Z + Mg++ = MgZ + 2Na+
Hard water contains Mg++, Ca++ (Na2O.Al2O3.SiO2.H2O)
After seven days, CaO. Al2O3.SiO2.H2O
Pre-treatment Process
The process is done to make the textile materials suitable for dyeing, & printing. it is known as pre-treatment
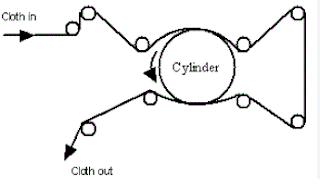
Singeing:
 |
| Fig: Singeing Process |
It is the process by which we remove protruded fiber from the fabric or fiber surface. It is basically for woven fabrics.
1. Plate Singeing Machine: In plate singeing, open fabric is passed upon the copper plate. To maintain every speed some draw & guide rollers are used. The temperature of a singeing plate is heating but the problem is that the temperature varies.
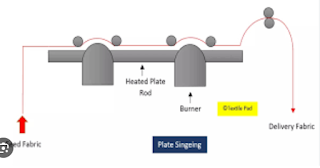 |
| Fig: Plate singing Process |
Advantages of Plate Singeing:
Due to friction between the cloth surface and the copper plate fabric surface becomes smooth.
Disadvantages of Plate Singeing Machine:
- Non-uniformly heat given
- Energy Losses
- Half Production
- Interstitches gap cannot be signed
- Both sides are not singing at a time
- Copperplate is not to be even. So, uneven singeing is performed.
Roller Singeing Machine
In a roller singeing machine rotating cylinder is used instead of a stationary curved plate as a plate singeing machine. It consists of cast iron or copper and is provided with an internal firing system.
- In this machine, the surface temperature of the cylinder is more uniform in all processes.
- High production
- Suitable for velvet and piles of fabric
- No energy losses
- The rotation of the cylinder is opposite to the fabric movement. So, singing efficiency is more effective
- Remove protruded fiber more effectively by two-cylinder
- Both sides of the fabric can be singed at a time.
Gas Singeing Machine
In this type of singeing machine, the fabric passes over a burning gas flame in such a way that only the protruding fiber burns and the main body of the fabric is not damaged by the flame. This is the most common type of singing machine. 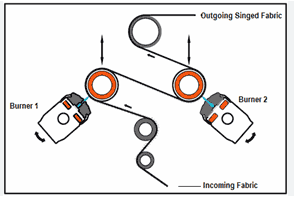 |
| Fig: Gas Singeing Machine |
- Uniform temperature ensures the proper singeing
- Both sides of the fabric can be singed
- As the process is continuous, there is no hamper in production
- All protruded fiber can be easily removed
- It is a standard process
- It ensures ideal singeing
- The fabric is interstitches of warp and weft yarns are singed.
Disadvantages of Gas Singeing Machine
- A dirty burner can produce spots on the fabric
- The process is not suitable for synthetic fiber
- Due to the inconsistent speed of fabric, it may be burnt
- Required high-skilled labor
- High maintenance needed
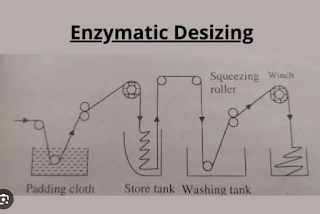
Desizing:
 |
| Fig: Desizing Process |
Desizing is the process of removing sizing material from the warp yarns of woven fabric. Sizing chemical consists of soluble starch material, a coupling agent, a lubricant, and a range of additives.
Desizing Method:
1) Hydrolytic
- Rot Steep
- Acid Steep
- Enzymatic Steep
2) Oxidative
- Chlorine
- Chloride
- Bromide
Objectives of Desizing:
- To remove the sizing material from the fabric
- To increase the absorbency power of the fabric
- To increase the affinity of the fabric to the dyeing chemical
- To make the fabric suitable for the next process
- To increase the luster of the fabric
Steeping:
It is the process where soaking in the liquid (water) of a solid to extract flavors or soften it
Rot Steeping:
In rot steeping, the steeping cloth is passed through warm water at a 40-degree temperature. For this cloth, it is squeezed up to 100% expression by a squeezed roller and kept in washing for 24 hours.
Advantages of Rot Steeping:
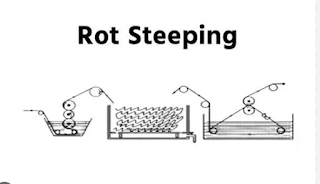 |
| Fig: Rot Steeping |
- Desizing agent warm water, no special chemical is used
- It is the cheapest and oldest method of steeping
- Time 24 hours
- Less weight loss
- The desizing process is good
- Less cost
Disadvantages:
- More empty space required
- Take about 24 hours
- Less production
- Hydrocellulose form
Acid Steeping
In acid steeping, fabrics are impregnated with a dilute sulphuric acid solution in a room where the temperature is 30 degrees and have to be it for 8-12 hours. Besides this, we have to remember that fabric should not be dried in the storage room. Otherwise, it may damage the fabric
- Take less time about (8-12) hours
- The desizing process is better
- Less space is required
- The most useful de-sizing process
- High production
- Starch fully liquified
Disadvantages:
- Dilute sulfuric acid is needed as a de-sizing agent
- Fabric may be damaged if it is kept after drying
- High-cost process
- More weight loss
- Hydro-cellulose form
Enzyme Steeping
In enzymatic steeping, the fabric is passed through a hot water vessel where the temperature is 40 C. Then it is transferred to the enzyme (0.5 - 2)% bath where (60-70)C temperature is needed and has to be it for 12 hours.
Advantages of Enzyme Steeping
- The safest method of resizing
- The desizing process is good
- Takes about 8 hours
- Desizing agent enzyme
- Less space is needed
- High production
- No problem with cellulose
Disadvantages
- High-cost process
Chlorine Desizing
In chlorine de-sizing, the fabric is dropped into a water vessel. Then it passes into a chlorine chamber where chlorine gas is traversed through the perforated bottom of the chamber. The chlorine gas reacts with fabric water and produces a small amount of dilute HCl and [O]. This incipient oxygen reacts with starch to remove this sizing material.
Advantages of Chlorine Desizing:
 |
| Fig: Chlorine Desizing |
- Fully reactive process
- High production
- Take time (2-3) minutes
Disadvantages:
- Need distilled water
- High maintenance
- Fiber degradation
- High cost
Estimation of Desizing Effect:
- Measurement of weight loss percentage i.e. we get the value of the weight of sizing material
- Tegawa solution = Iodine, potassium iodine, methanol
It is used to indicate the amount of sizing material remaining in the de-sizing fabric
When we fall one drops tegawa solution into the fabric after resizing. If it shows a lighter color in the water. Then, indicate no sizing material remains in the fabric. If it shows a darkish color then it indicates there is sizing material remaining in the fabric.
Estimation of Desizing Effect:
- Measurement of weight loss %
- Tegawa solution (Iodine, Potassium Iodine, Methanol) - It is used to indicate the amount of sizing material remaining in the desizing fabric)
Qualities of Silk Can be Classified as:
- Ecru - (2-5)% sericin remove - scoured in weak soap solution
- Sople- (8-15)% sericin remove - 10% soap solution at (30-35)C for (1-2)hours
- Boiled off- (30% sericin removed - completely degummed- (10-15)% soap solution at (90-95)C for (1-3)hours
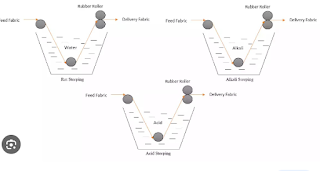
Scouring
 |
| Fig: Scouring Process |
Scouring is the process of removing dust and impurities from the fabric. Dust is created from the machine or naturally. These impurities are hydrophobic in water.
Scouring Process
Fat/Oil Converts
- Saponiable + NaOH = Soluble Soap
- Unsaponifiable + Solvent + Detergent = Emulsion
Chemicals Used in Scouring
- Detergent
- NaOH
- Solvent
- Sequestering Agent
Objectives of Scouring
- To remove the natural or added impurities like oil, wax, gum, and fatty materials as completely as possible
- To get a clean and even fabric suitable for the next process
- To increase the absorbency of the fabric
Effects of Scouring
- Saponiable oils are converted into soap
- Unsaponiable oil and waxes are emulsified by soluble soap from saponiable oils.
- Dust particles are removed and held in a stable suspension form
- Mineral matter is dissolved
- Proteins are hydrolyzed into soluble degradable products
Scouring of Cotton
The cotton cellulose material is treated with a solution containing an alkali, detergent, caustic soda, a sequestering agent, a wetting agent for the removal of proteins, coloring matter, pectin, and waxes at high temperature
Cellulose- 94%
Proteins - 1-1.5 %
Pectin - 1%
Mineral - 1%
Wax - 0.5 %
Coloring matter - 2%
Recipe Required for Scoring
Sequestering Agent - 1cc/L
Alkali (NaOH) - 3mg/L
Na2CO3 - 5gm/L
Detergent/ Wetting Agent - 10cc/ L
Temperature - Boiling
pH - 11
Time- 30 minutes
Reduction of Removal of Impurities During Scouring
Oil, fat, and waxes + alkali = Water soluble soap
Pectin Substances + NaOH = Soluble salt of practice acid
Protein + NaOH = Soluble amino acids
Lubricant and coloring matter = Water soluble products
Scouring of Silk
The scouring of silk is the degummed process used to remove the sericin from the fibroin. Sericin is the gummy element that keeps together with fibroin floss and gives the silk a hard and dull appearance. So we have to remove this sericin from silk by treating it with a soapy solution or buffer-dissolving agent and enzyme
Silk with sericin + Soapy solution/Buffer dissolving agent + enzyme = Silk
The treatment causes a loss of weight ranging from (24-28)% and gives the degummed silk a lustrous appearance.
Fibroin - (70-80) %
Sericin - (20-30) %
Carbohydrate - (1.2-1.8) %
Wax - (0.2- 0.4)%
Inorganic matter - 0.7%
Pigment - 0.2 %
Scouring of Wool
Yarn/fabric with oil and dirt/contaminants + Na2CO3/NH3/Surfactants + Soluble soap = Wool
which carries out a softer washing to avoid any damage to the fiber
Wool as received contains about 30 to 40 % impurities consisting of sheep natural oils, dirt, suit, sand seeds, etc.
It is necessary to remove these impurities for good dyeing
Impurities of wool are given below:
Wool fiber -33%
Suint - 28%
Fat - 12 %
Dirt - 26 %
Mineral water - 1%
Chief Method of Wool Scouring
1. Emulsion Scouring:
Although wool fat is difficult to saponify when it is treated with (2-4)% soap & 2% Na2CO3 at (40-45)C temperature. It provides minimum damage and maximum scouring. It is the most common method of wool scouring
2. Suint Scouring:
a) Desuinting:
Dusted wool + liquor + Cold suint + (160C) = Sedimentation / Centrifuging wool
b) Grease Removal:
Centrifuging wool + Clear suint solution + (60C) = Grease free + pH(4.5-5.5) & 70C = Suint liquids free wool
c) Rinsing:
Suint free wool + 0.1 % soap + 38 C = Wool
d) Soaping:
Wool + 0.3 % soap + 41C = Wool
e) Rinsing:
Wool + 0.1 % + 32C = Wool
- This scouring is very economical
- It gives brighter and whiter wool
- It is more open and lofty than the soap-scouring
Wool with dirt & suint + Solvent (Benzene,CCl4) = Wool remove (0.5-0.75) % fat
Wool with solvent + Blowing warm air through Kier m./c + Condenser = Wool + Solvent
Extracted wool with still contain suit + Warm water (special bowl/unstinting machine) = Wool
- Removal of oil and grease from the wool
- Gives material of superior quality, strength, and softness
- More suitable for carding
- Less fiber degradation
Removes (10-18)% weight of greasy wool vegetable matter is removed to a large extent by the milter scouring process. Wool has a softer handle and lofty appearance with better color.
Scouring of Synthetic Fibers
To remove oils, lubricants, anti-static substances, dirt, and contaminants from the synthetic fiber. It should be carried out with surfactants, detergents, and emulsifying agents.
Function of Used Chemicals in Scouring
Alkali - It converts the oil into water-soluble fatty acid and soap
Wetting agent/ Detergent - It reduces the surface tension between the material and soap. It also helps to clean the material
Sequestering Agent- It reacts with metallic ions (Mg++, Ca++, Fe++), etc. producing metallic compounds by which it removes the hardness of the water.
Estimation of Scouring Process
The measurement of Weight Loss Percentage:
There are three types to estimate the rate of scouring process
- Water drop absorption
- Sinking time test
- Capillary rise method
1. Absorbency Test: In this method, a drop of water is placed on the dry-scoured fabric. If the water drop is absorbed within (3-5) seconds then it is considered that the scouring process is efficient. If it takes (2-3)minutes to absorb water then the scouring process is now good and there is a need to repeat the scouring process
a. Drop Test: If we drop water on the scoured fabric if it is stable and forms an uneven circle above the fabric, it indicates less scouring. If it gives an even circle above the fabric, it's good scouring.
b. Wicking Test: We we immersed scoured fabric with specific length & width for 5 minutes. This test is determined by the amount of absorbing water from the fabric. The range of scrouing quality is given below.
Scouring < 30mm - Not Good
Scouring (40-45)mm - Good
Scouring > 50mm - Excellent
2. Immersion Test:
In this method, we immersed 1" length and 1" width scoured fabric. It indicates scouring by immersing time of the scoured fabric. This method also needed a particular amount of water.
Bleaching
 |
| Fig: Bleaching Process |
Bleaching is the process of improving the whiteness of the fabric and removing the coloring material from the fabric with minimum degradation.
Objectives of Bleaching
- To ensure a high degree of whiteness for white goods
- To ensure even and stable white for dyed goods.
- To ensure minimum tendering of the fiber
- To increase the absorbency for the further process
- Completely removal of cotton seeds and motes
Recipe Required for Bleaching
Sequestering agent - 1cc/L
Hydrogen peroxide - 15% (Weight of fabric)
Stabilizer (Na2SiO3) - 1/3 of H2O2
NaOH - 3gm/L
Na2CO3 - 5gm/L
pH - 11
Temperature - Boiling
Time - 30 minutes
M: L - 1:80
Properties of Universal Hydrogen Peroxide in Bleaching
- Colorless liquid
- Minimum degradation of fiber in bleaching
- Relatively cheap
- Stable under acid pH
- Activated under alkaline conditions
- Can be employed in all types of fiber
- It requires minimum manipulation of fabric and less labor
- Less weight loss
- Less water needed
- Have more absorbency power
- Can be used in both scouring and bleaching processes in one operation.
Mechanism of Hydrogen Peroxide Bleaching
Hydrogen peroxide (H2O2) is a very weak acid. It could be ionized to form per hydroxyl ions (HOO-)
H2O2 = HOO- + H+
The formation of the active perhydroxyl ions occurs by alkaline conditions and these anions are a source of active oxygen that has the bleaching effect.
H2O2 + OH- = H2O + HOO-
HOO- = OH- + O
Several competitive reactions occur in the presence of iron, cobalt, copper, manganese, and its oxide and ions. They act as catalysts for the decomposition of hydrogen peroxide. In the presence of the catalyst, there is a direct breakdown of hydrogen peroxide to water and molecular oxygen.
2H2O2 = 2H2O + O2
The breakdown is most rapid in alkaline conditions. The molecular oxygen wants to escape from the bleach solution reducing the bleaching effect and can cause damage. So, a stabilized is added to hold the fabric over alkali.
Mercerization
 |
| Fig: Mercerization Process |
Mercerization is a physio-chemical process where the cotton goods are treated with (15-25)% sodium hydroxide solution at a temperature of (20-30)C. It is necessary to hold the fabric under tension and wash it thoroughly while it is under tension.
Physical Changes After Mercerization
- Length-wise shrinkage and swelling axially
- Untwisting of the fiber
- Changes of cross-section
- Increase tensile length
- Decrease the tension of the break
- Increase the luster
Chemical Changes After Mercerization
- Breaks hydrogen bonds and weak van der Walls forces
- Formation of soda cellulose
- Increase affinity to dyestuff and chemicals
- Increase hygroscopicity
Reason for Chemical and Physical Changes in Mercerizing
Swelling and Shrinkage of Cellulose: When cellulose is immersed in a solution of caustic soda the material swells. The fiber quickly turns to untwist from its twisted ribbon.
The cross-section of the fiber diminishes, and the diameter of the fiber becomes more round. The surface of the nearly cylindrical cotton after mercerizing reflects more evenly to all sides than the kidney-shaped cotton and the fiber surface becomes more lustrous.
Structural Modification: Due to the mercerization of cellulose many hydrogen bonds are broken, the plane of molecular chains moved apart, and the molecular structure tends to become recrystallized. The chains within the cellulose structure become more uniform and the chains are given a slight twist.
Increase Lustre: The cross-section of the unmercerized cotton is irregular and ear-shaped. While the lumen is irregular and resembles a collapsed tube. All these factors result in less luster.
In mercerizing, the cross-section of cotton by swelling becomes circular and the lumen is practically eliminated. Lustre also depends on reducing the axial ratio and increasing the light scattering within the fiber.
Gain In Strength: Mercerization increases the tensile strength of cotton fibers by eliminating the weakest point in the fiber.
Increased Moisture Absorption: Due to caustic soda penetration, many hydrogen bonds are broken and hydroxyl hydrogen bonds are increased by about 25 % mercerization.
Thus, increases in the amorphous content of the fiber increase the proportion of the amorphous region is directly related to moisture absorption.
Increase Dye Absorption: Mercerized cotton increased rate of dyeing due to neps and unripe cotton is less prominent. Generally immature cotton with a large lumen responds particularly well to increased light scattering and hence decreased dye uptake. Dead fiber does not contain dye in the same amount as mature.
Increased Reactivity: It also accelerates the reaction with acids and oxidizing agents and is susceptible to degradation.
Removal of Immaturity: It is also used to remove immature fibers to obtain a level of dyeing effect on cotton fabrics.
Physical Compactness: It increases the dimensionality of woven fabrics. increasing temperature can be said to improve mercerization because when the goods are bleached, then the mercerized goods are dense.
Some Effects of Mercerization:
Lustre: The shape of silk or viscose is cylindrical. Cotton has the structure of a flattened tube with several twists. When the light falls on cotton fibers gets reflected irregularly and as a result, there is no lustre in cotton fibers. (Ear-shaped Cross Section)
After mercerizing, the ear is shaped to flattened tube cotton becoming smooth and more regular in surface structure. This results in increasing the luster of the fiber (light reflects regularly or more evenly)
 |
| Fig: Physical and Chemical Changes Effect on Mercerization |
Affinity to Dyestuff and Chemicals: NaOH acts as a decrystallising agent which increases the amorphous region in the fiber and increases its affinity to dyestuff and chemicals. Due to the swelling of the fiber, molecular orientation is increased. The cross section of the fiber becomes more uniform and regular which results in even dyeing with better color.
Tensile Strength: When the cotton fiber, yarn, or cloth is mercerized, its tensile strength increases by 10-50 %.
Mercerization under tension assists the cotton polymers in aligning themselves in a regular way which increases hydrogen bond formation which is responsible for the additional strength of the fiber.
Besides this, lengthwise shrinkage of the polymer chain minimizes the weak link in the fiber.
Swelling of Cotton After Mercerizing:
Mercerization is based on the swelling action of NaOH on cotton. In cellulosic fiber, There are three non-polar hydroxyl groups where some form hydrogen bonds and others are non-bonded.
In mercerization, cotton is treated with 24% caustic soda solution. These solutions contain the dissolved sodium hydroxide as Na+ and OH- ions. Where cotton is entered into a caustic soda solution. The Na+ and OH- can easily diffuse in the amorphous region, where these molecules try to vibrate with greater amplitude. As a result, some hydrogen bonds and other weaker bonds of the crystalline region are ruptured and unbound hydroxyl tries to vibrate with the same amplitude. Then, further, NaOH can diffuse and get bound to hydroxyl groups.
Dyeing Process:
 |
| Fig: Dyeing in Textile Industry |
Dyeing is the process of applying color to the fabric with the help of dyes and chemical auxiliaries. Dyeing is also the process of coloring textile materials by immersing them in an aqueous solution dye, called dye liquor. Normally the dye liquor consists of dye, water, and auxiliary. To improve the effectiveness of dyeing, heat is usually applied to the dye liquor.
Classification of the Dyeing Process
There are three types of dyeing processes used in the textile industry.
1. Exhaust/Batch Dyeing
- Winch
- Jet
- Jigger
- Beam
2. Semi-continuous Dyeing
- Pad-Batch
- Pad Steam
- Pad Dry
- Pad thermos
Exhaustion:
The exhaustion is defined as the mass of dye taken up by the material divided by the total initial mass of dye in the bath but for a constant volume.
Exhaustion % = C0-Cs/C0 *100%
C0= concentration of dye bath initially
Cs= concentration of dye bath at some point during the dyeing process
Percent exhaustion continues to increase as the concentration of the dye in the liquor becomes greater.
Dyeing at a low liquor ratio decreases the amount of dye wastage. It also consumes less water and steam and allows an even salt concentration with less added salt.
Dyeing in low liquor ratio is an important factor in economizing in dyestuff consumption.
For Producing heavy shades the amount of dye becomes greater if the m/c requires a high ration of liquor for goods
Heat Setting
 |
| Fig: Heat Setting in Textile Industry |
Heat Setting is the process for fabrics made of synthetic fibers, for triacetate, and partly for PAC fibers. Since it grants excellent dimensional stabilization and crease-proof properties, maintain till the fabric is exposed to temperatures exceeding the heat setting one (after being treated with water at a temperature above in the second order glass transition temperature.
Heat setting is carried out on grey fabrics and dyed fabrics.
The process grants excellent dimensional stability and good crease-proof properties.
As far as operating conditions are concerned, the fabric must be treated in accurately controlled moisture and temperature conditions.
Printing
Printing is described as localized dyeing. Dyes or pigments are applied locally or discontinuously to produce the various designs. Printing is the production of all active designs with defined boundaries made by the artistic arrangement of a motif in one or more colors.
 |
| Fig: Printing Process |
Method of Printing
There are five types of printing methods in the textile industry.
- Digital Printing: Ink Jet Printing
- Heat-transfer Printing: Design transferred to fabric from specially printed paper by heat & pressure
- Electrostatic Printing: Prepared screen covered with powdered dye, passes through electric field & pulled onto the material
- Foil Printing: Adhesive applied to fabric, foil heated on heat transfer press & bonds to adhesive pattern
- Stencil Printing: Separate pattern cut for each color, color is applied in a thick paste or sprayed on with an air gun
Finishing in Dyeing
Finishing is the final process given to a textile material to give a good appearance, desirable feel, stiffness, softness, wash, and wear finish, water-repelling finish, fireproof finish, etc. It also provides some desired functional properties.
 |
| Fig: Finishing Process |




Comments
Post a Comment