VAT Dyes: Properties, Applications, and Benefits
What is Vat Dye/ Vat Dye Definition
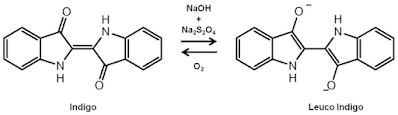
This chapter discusses vat, indigo and sulphur dyes together because their use in dyeing involves the same principles. Vat dyes are one of the oldest types of dye Vat dyes in particular give dyeings on cellulosic fibres with the best overall fastress properties. Because of the popularity of blue jeans, Indigo is still one of the most important of all dyes in present use. Natural Indigo was obtained by extraction from leaves, and by fermentation in wooden vats, the origin of the term 'vatting Today, Indigo is synthesised from manufactured intermediates. Its application involves reduction to the water-soluble leuco compound, dyeing the cotton and re-oxidation of the leuco dye in the fibres to the insoluble pigment, The basic steps involved in vat dyeing
Vat dyes are water-insoluble pigments. They are called dyes because the chemical reduction in alkaline solution converts the pigment into a water-soluble leaf form with substantivity for cotton. The vat pigment and the leuco compound often have quite different colours - blue and pale yellow in the case of Indigo- and the progress of the reduction is often easy to observe. After dyeing with the lead compound, the pigment is regenerated in the dyed cotton by oxidation. The overall process therefore involves three key steps:
(1) reduction of the pigment to the soluble leuco compound, a process called vatting:
(2) absorption of the leuco compound by the cotton during dyeing.
(3) oxidation of the absorbed leuco compound in the cotton, reforming the insoluble pigment inside the fibres.
The use of strongly alkaline solutions (pH 12-14) for vatting and dyeing limits the use of most vat dyes on cellulosic fibres.
CHEMICAL CONSTITUTION OF QUINONE VAT DYES
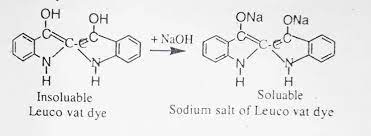
In the case of Indigo, vatting involves reduction of the a pair of conjugated carbon groups (Figure 1.4). For most vat dyes, these carbonyl groups are present in an anthraquinone or polycyclic quinone. The parent quinone is an insoluble pigment. The leuco compound is the sodium salt of the diphenol or hydroquinone formed by reduction in NaOH solution (2). It is reasonably soluble in water, substantive to cotton and easy to oxidise back to the red pigment Reduction is carried out in a strongly alkaline solution to avoid the formation of the vat acid (3). This is the free diphenol formed by protonation of the anionic leuco derivative. The vat acid is malleable in water, has no substantivity for cotton and is more difficult to oxidise
 |
| Ci Vat Red 42 |
 |
| leuco compound formed by reduction |
 |
| vat acid formed by protonation of the anionic leuco derivative |
 |
| CI Vat Blue 4 |
Quinone vat dyes are usually either anthraquinone derivatives (1) or polycyclic quinones. Indanthrone (4), the first quinone vat dye, was discovered by accident during attempts to make an Indigo-type derivative from 2-aminoanthraquinone The structures of most vat dyes are often quite complex and have no ionic groups. Many of the polycyclic derivatives have no attached substituents, but a few have halogen, hydroxyl, methoxyl or amide groups. The more complex dyes may have several reducible quinone groups
The molecular structure of a vat dye influences the water solubility, substantivity and rate of diffusion into the cotton of the leuco compound used in dyeing. A classification of quinone vat dyes according to dyeing characteristics is more useful for our purpose and will be given later in this chapter.
THE REDUCTION OF QUINONE VAT DYES
Vat dye dispersions
Quinone vat dyes are available as fine powders or grains and as liquid dispersions or pastes. The insoluble pigment is extensively milled with dispersants such as sodium lignosulphonates to produce very small particles (Section 15.3). Typically, at least 50% of the particles will have diameters less than 1 um. All forms must disperse readily in water and any aggregates of particles must easily break up to give a fine, stable dispersion. The rate of reduction to the leuco form is usually faster, the finer the pigment dispersion.
Solid forms contain much dispersing agent and more may be added to the dyebath to keep any unreduced dye in suspension. The addition of The additionydrosulphite (dithionite) (Na, S, O,) to the alkaline aqueous dispersion of the vat dye brings about rapid reduction. In pigmentation dyeing methods, the fine pigment particles are uniformly deposited in the goods by padding or liquor circulation before reduction occurs. Very fine dispersions are needed for these
pigmentation processes and the proportion of fine particles is higher than for what pigments in the traditional vetting process. Dye powders for pigmentation dyeing have higher dispersant content. Partes and liquid forms are useful for printing and continuous dyeing. They will usually have less dispersant but contain appreciable amounts of water. Some ranges of dyes are deliberate mixtures of water and dispersed dyes for dyeing cotton/polyester blends
Reducing VAT dyes with hydros
Reduction of quinones is not easy and vatting requires a strong reducing agent The dihydrate of sodium dithionite, or hydrosulphite. Na2S2O4.2H, O. is used almost exclusively and goes by the common name of these. Vatting U came out under strongly alkaline conditions for several reasons
(1) The reduction of the quinone (QO2) with hyds to give the leuco dye (QO2-), and the oxidation of the hydros by oxygen, consumed alkali.
(2) It is important that the insoluble vat acid does not precipitate
(3) Hydros is unstable in solution, particularly under acidic or neutral conditions and at higher temperatures. Its decomposition forms thiosulphate and bisulphate. This decomposition reaction consumes hydroxide ions and it is faster as the solution becomes more acidic
QO2 + Na2S204 + NaOH = QO2- + 2Na+ + 2Na2SO3 + 2H2O
Na S₂O + 2NaOH+ 0₂ = Na2SO3 + Na₂SO4+ H₂O
2Na2S204 +2NaOH = Na2S2O3 + 2Na2SO3+ H₂O
The decomposition of hydros is exothermic. Decomposition of solid Na2S2O4.2H, O can lead to spontaneous ignition. Cool, dry conditions are therefore necessary for storage. The decomposition of hydros occurs rapidly in an acidic solution. Alkaline hydros solutions are more stable provided oxygen is absent;
Because hydros reacts readily with oxygen (Scheme 17.1), the material being dyed with the leuco dye should not be over-exposed to air. Oxidation of the excess hydros can lead to subsequent oxidation of the leuco dye and precipitation of the quinone, giving uneven surface deposits of pigment on the goods. A significant excess of hydros in the bath is usual. The amount used depends on the dyes and the type of machine. The suppliers give suitable recommendations for their shades. Other more stable reducing agents, such as sodium sulphoxylate-formaldehyde (HOCH, SO, Na), are used in printing with VAT dyes to ensure the stability of the pastes. Steaming of the printed material activates this reducing agent and the vat dye in the print paste is reduced. Sodium sulphoxylate-formaldehyde is a poor reducing agent at 25 °C and is more stable than hydros Other reducing agents such as thiourea dioxide and glucose are now being suggested as more environmentally friendly alternatives to hydros.
anthraquinone type vat dyes and indigoid vat dyes give much paler yellow to brown leuco compound solutions. The vatting process is also slower and requires less alkali.
Vatting
Traditional vatting involves adding hydros to a fine, relatively concentrated aqueous dispersion of the vat dye pigment containing NaOH. The vatting temperature is often higher than the subsequent dyeing temperature. This gives rapid reduction. The concentrated solution of the leuco form is then diluted by adding it to water in the dye bath. The bath water also contains NaOH and some hydros to avoid any oxidation from dissolved atmospheric oxygen. Vatting may also be carried out directly in the dye bath if the dye is readily reduced.
Dyeing takes place at temperatures lower than the vatting temperature to avoid decomposition of the hydros. This also decreases the risk of over-reduction of polyquinone dyes such as Indanthrone. Over-reduction is the reduction of more than one pair of conjugated carbonyl groups in a polyquinone and often results in poor colour yields and off-shade dyeings. Reduction inhibitors, such as sodium nitrite or glucose, prevent over-reduction during vatting. Anthraquinone and polycyclic vat dyes give leuco solutions with intense colours, often very different from those of the original pigment dispersion. The vat dye flavanthrone is a yellow pigment that produces a deep blue vat. It is often used in paper strips for the detection of excess hydros.
Vatting conditions for quinone vat dyes vary widely. Three main types of vatting process are used depending upon the dyes being used. These involve
(1) a concentrated NaOH solution and a high vatting temperature (60 °C);
(2) a moderate concentration of NaOH and a lower Vatting temperature of 50C
(3) a low concentration of NaOH and a low vatting temperature (30-40) C
THE SUBSTANTIVITY AND DYEING CHARACTERISTICS OF VAT DYES FOR CELLULOSIC FIBRES
Basic steps in the dyeing process
The dyeing of cellulosic materials with quinone vat dyes follows a four-step sequence:
(1) preparation of the vat containing the leuco forms of the dyes;
(2) dyeing of the material, in which the fibres absorb the water-soluble leuco compound;
(3) oxidation of the absorbed leuco compound back to the parent pigment inside the fibres;
(4) Soaping of the dyed material to remove pigment loosely adhering to the fibre surfaces and to develop the true shade and fastness properties.
Classification of vat dyes
For quinone vat dyes, there is no single classification according to dyeing properties as is the case for the direct dyes. The German Interessen Gemeinschaft für Farbenindustrie (IG) developed one popular classification for their
Indanthrene range of vat dyes based on leuco compound substantivity and the required dyeing conditions.
There were three main types:
(1) The IN (indanthrene normal) group of dyes require the use of concentrated NaOH and high vatting (60 °C) and dyeing temperatures (60 °C). No salt is added to the dyebath because of the high substantivity of the leuco dyes for cotton;
(2) The IW (indanthrene warm) group of dyes require only moderate amounts of NaOH and lower vatting (50 °C) and dyeing temperatures (50 °C). The leuco forms of these dyes have moderate substantivity for cotton and some addition of salt is needed during dyeing to aid exhaustion;
(3) The IK group of dyes only need a low concentration of NaOH with low vatting (40 °C) and dyeing temperatures (20 °C). These dyes have low substantivity for cotton and need considerable salt for good dyebath exhaustion. Some have amide groups that would be hydrolysed under the vatting and dyeing conditions used for IN and IW dyes.
There are special processes for some black vat dyes that require an oxidative after treatment to develop the full black colour. Table 17.1 compares the characteristics of these three types of vat dye. The required concentrations of hydros, caustic soda and salt increase with increasing amounts of dye in the bath and with increasing liquor ratio.
Table 17.1 Characteristics of the three main types of vat dye
Vat dye type
Conditions IN IW IK
NaOH concentration (g 1-¹) 4.5-7.5 2.0-4.0 1.5-2.5
NaCl concentration (g 1-¹) 0 5-25 10-40
Dyeing temperature ("C) 50-60 45-50 20-25
Vatting temperature (C) 50-60 45-50 40
There are various other classifications of vat dyeing methods. The SDC recommend tests to determine the best dyeing method [1]. In this, the colour strengths of dyeings produced under different dyeing conditions are compared with those of standard dyeings using a grey scale. This test applies only to anthraquinone dyes. There are also SDC tests for determining the strike. migration and levelling characteristics of vat dyes. Different companies have different classification systems for their vat dyes. Because vatting and dyeing conditions vary from one dye to another, the suppliers' recommendations should be consulted.
The high substantivity of leuco vat dyes
The origin of the substantivity of leuco vat dyes for cotton is far from clear Hydrogen bonds between cellulose hydroxyl groups and phenolate ion groups, or amino or amide substituents, in the leuco dye may be involved in dye-fibre binding. The substantivity of the leuco dye is very dependent on the position of the phenolate ion groups and on the actual molecular structure. Substantivity tends to be greater the larger the surface area of the leuco dye molecule and van der Waals interactions between the dye and fibre are important. Molecules of leuco vat dyes tend to be large and, although coplanarity of the structure is essential, the structures are often not as long and linear as for direct dyes. Although the planar molecular structures of leuco vat dyes should promote aggregation, there is not much evidence of this in dyeing solutions.
In the dyeing process, the influence of salt addition and increasing dyeing temperature are similar to the effects found in dyeing cotton with direct dyes. Unlike direct dyes, however, dyeing with many leuco vat dyes is very rapid at relatively low temperatures and usually gives high exhaustion at equilibrium. The ability to control the dyebath exhaustion by salt addition is very limited since the bath initially contains a high concentration of sodium ions from the added hydros and caustic soda. Because of this, many dyes have a rapid strike. The debate may be 80-90% exhausted within 10 minutes. Because there is little diffusion into the fibres within such a short period, there is a strong tendency for cotton yarns and fibres to be ring-dyed in the early stages of dyeing. Diffusion only becomes important well after the time of half dyeing. The high strike must be controlled to avoid unlevel dyeing since the low dyeing temperatures do not favour migration The behaviour of the leuco dyes is assessed by the usual migration and strike tests in comparison with the behaviour of standard dyes [1]. The dyeing methods recommended correspond to the IN, IW and IK methods described. The rate of diffusion of a typical leuco vat dye into cotton is about half that of direct dye at the same temperature. The high rate of dyeing of leuco vat dyes is therefore a consequence of their high substantivity for cellulose. Since the standard affinities of leuco vat dyes are no higher than those of direct dyes, the
high substantivity and rate of dyeing are caused more by the high electrolyte content of the dyebath than by high inherent affinity. The initial strike can be retarded using polyethylene oxide complexing res The initially formed dye-auxiliary complex presumably slowly breaks down and gradually liberate free leuco dye, thus controlling the initial rate of adsorption Both non-ionic and cationic retardants are used but these decrease the exhaustion. They can also be used for levelling of uneven dyeings. Soch dying auxiliaries should be added to the dyebath after vatting. The dye manifester's recommendations should be consulted since these agents are often dye-specific. Similar chemicals can be used for stripping faulty dyeings. This is heavy because of the high substantivity of the leuco vat dyes and the stripping auxiliaries send form much more stable complexes with the leuco dyes than those obtained from levelling agents.
DYEING COTTON WITH LEUCO VAT DYES
Preparation
As always, good preparation of the goods is essential to avoid a non-uniform distribution of residual impurities that might hinder level dyeing or that might interact with the dyes. Water quality is important since the calcium and magnesium salts of some leuco dyes may precipitate if hard water is used. The we of sequestrants or protective colloids is often recommended.
Dyeing conditions
Quinone vat dyes are used on all forms of cellulosic materials. The vat is all pre-prepared and added to the dyebath containing the goods, possibly in successive portions. Vat dyes of high substantivity are often used for dyeing deeper shades where the effect of poor levelling is less visible. They usually follow a Freundlich adsorption isotherm (Section 11.1.1) so that using more dye
promotes deeper shades but with diminishing returns. The dyeing temperature may be increased to promote migration if the dye is stable. This gives better penetration into tightly constructed materials. Subsequently cooling the dyebath p better exhaustion. Any required salt may be added in portions towards the end d the process for dyes of lower substantivity.
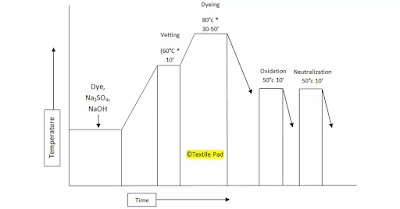
The liquor ratio is usually in the range of 10:1 to 20:1. The actual an of caustic soda and hydros required for vatting, and for addition to the dacha depend on many factors, such as the dyeing temperature and time, and particularly on the degree of exposure to air during dyeing. The latter is mainly a character of the particular dyeing machine used. As in dyeing cellulosic fibres with some direct dyes, the rate of dyeing with leuco vat dyes is higher at higher temperatures lower liquor ratio and lower dye concentration. It is not much affected by the relatively high NaOH and hydros concentrations. Many leuco compounds have high substantivity and level dyeing requires careful control of the dying temperature, the total salt concentration and the gradual addition of the concentrated leuco dye vat to the dyebath. Figure 17.4 shows a typical procedure for dying cotton with a quinone vat dye. amount
Problems with anthraquinone dyes.
Several chemical problems arise with some quinone var dyes. These include
(1) Multiple reduction steps for poly quinones such as indanthrones
(2) isomerism of leuco compounds to xanthones,
(3) hydrolysis of amide groups;
(4) over-oxidation after dyeing
(5) dehalogenation of some dyes.
To minimise these types of problems, the supplier's recommendations for voting and dyeing must be followed.
Indanthrone (CI Vat Blue 4) and some of its derivatives show several disease problems. Indanthrone has two anthraquinone residues in its molecule. The m blue leuco compound used in dyeing corresponds to the reduction of one of the anthraquinone groups (5, in Figure 17.5). If both anthraquinone groups are reduced, the final product (6) gives a brownish yellow solution, pe substantivity for cotton and is more difficult to residue. Such over-refin produces duller blue dyeings of lower colour yield. Indantimne sales one of the quinone vat dyes whose leuco compound will tautomerase via the mat sed ox anthrone (7) if the concentration of NaOH in the solution is not sufficiently g Oxanthrones are not easily oxidised to the parent quinine and therefore results in dyeings of poor colour yield. Mild oxidation of learn var dyes of the Anthraquinone type with hypochlorite produces a much greenery derivative
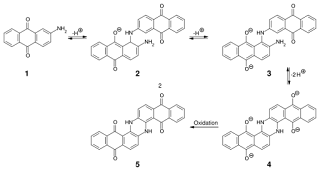 |
| Ahthraquinone Vat Dyes |
This is responsible for the rather poor chlorine fastness of indanthrone. The test is reversible and treatment of over-oxidised dyeing with a dilute alkaline hydro solution followed by air oxidation regenerates blue Indanthrone.
Partial formation of the green azine derivative can occur during air oxidation if the fabric still contains a relatively high concentration of NaOH solution, Rising out the excess alkali from the dyeing is therefore necessary before significant oxidation by oxygen has occurred. Some of the chlorinated indanthrone vat dyes are prone to elimination or substitution of a chlorine atom unless vatting is carried out with care. Although the indanthrone-type dyes represent rather special cases it is obvious that vatting of the insoluble pigment to produce a solution of the leuco derivative for dyeing is not always a simple operation. It is important to follow the supplier's recommendations for all stages of the dyeing process:
Aftertreatment of the dyeing
Once the actual dyeing process is complete, the goods go through a series of operations:
(1) rinsing to remove adhering exhausted dye liquor and chemicals. A reductive rinse with dilute alkaline hydros solution may be needed for heavy shades,
(2) oxidising the leuco dye to the pigment form using hydrogen peroxide and acetic acid;
(3) soaping at or near the boil. For soaping in a packaging machine, the first step may be dispersion at 60 °C to remove loose surface colour, followed by soaping at or near the boil. This is important to avoid filtration of eliminated dye particles by the package:
(4) neutralising the dyeing with acetic acid solution and final rinsing,
These processes are an essential part of the overall procedure and must be correctly carried out if the dyeing is to have the optimum fastness properties. The oxidation and soaping stages are discussed in the following section.
OXIDATION AND SOAPING AFTER DYEING OF VAT DYES
Oxidation
An oxidising agent has a much more positive redox potential than the compound that it can oxidise or, considered from the opposite perspective, the compound being oxidised has a more negative redox potential than the oxidising agent that it will reduce. Quinone vat dyes are generally difficult to reduce and say to ride For sulphur dyes the reverse is true. A typical vat de had potential around -0.80 V and oxidising agents with redox potentials more positive than about -0.50 V will in principle give complete oxidation of the local dye to the quinone. The rate of oxidation must also be acceptable Oxidation of a leuco vat dye can be achieved by exposing the goods to air, after an initial rice to remove adhering liquor, and therefore appears quite simple Air oxidation, however, is of little use for compact goods such as yarn packages, and is quite s often requiring 30-40 min for complete oxidation. It may also result in de-reactions and off-shade dyeings. Chemical oxidants are therefore generally preferred because they give more rapid and uniform oxidation throughout the material. Sodium dichromate, hydrogen peroxide, or sodium perborate or percarbonate are used at up to 40 "C. It is important that the initial adation is carried out under alkaline conditions to avoid forming the vat acid, which is much less soluble and hard to oxidise. Over oxidation of some dyes, such as Indanthrone, leads to changes in the hue of the dyeing (Section 17.5.3).
Soaping
Soaping removes pigment deposited on the fibre surface that would wash or rub off in use. The deposit is formed by oxidation of the leuco dye in the solution adhering to the fibres after dyeing. Thorough rinsing after dyeing, using a weak solution of alkaline hydros, helps to minimise this problem. Soaping involves thoroughly washing the goods in a detergent solution at or near the boiling point. It improves the light, washing and rubbing fastness of the dyeing Some vat dyes give a slight shade change on soaping so a standardised procedure is necessary for exact colour reproducibility.
The soaping process probably involves the re-crystallisation of the dispersed var dye particles obtained from the initial oxidation of the leuco dye. This is not a spontaneous process and requires heat and water. The growth of the pigment crystals inside the cotton fibre is limited by the pore size. It is this crystallisation process that is responsible for the slight change in hue and the improvements in the fastness properties.
PRE-PIGMENTATION DYEING METHODS
In many cases, leuco vat dyes have too high a substantivity for continuous dyeing and preferential absorption by the cotton fibres would result in exaggerated railing
The pigment form, however, has no substantivity for cotton at all. The pre-implementation dyeing methods are simple processes in which a fine dispersion of the vat pigment is uniformly circulated through the goods that are then treated with an alkaline byres solution to cause a reduction. Dyeing takes place around each original pigment particle with almost no migration. The dyeing is subsequently oxidised and soaped us before. Either of the two operations, the initial pigmentation or the reduction may be batch or continuous processes. This leads to a variety of possible dyeing methods, some of which are described below,
Precand semi-pigmentation batch dyeing methods
In the pre-implementation method, a fine dispersion of the var dye pigment is first circulated through the goods. In the second step, reduction of the leuco dye is carried out using NaOH and hydros. The latter may be added all at once or gradually. This method is useful for light and medium shades where levelling and penetration may be a problem in dyeing with a fully reduced VAT dye. It is. however, less suitable for compact forms of material. In package dyeing, the pigment dispersion must not be filtered out in the packages but rather gradually deposited into the package by salt addition that causes the particles to slowly flocculate.
In the semi-pigmentation process, all the chemicals are circulated through the goods at room temperature before gradual heating to start reduction. The rate of varting is very slow at the low starting temperature. As in the pre-pigmentation method, a fine dispersion is required.
The vat acid process is another alternative for package dyeing. After reduction of the vat pigment, in alkaline solution, the leuco vat acid is precipitated in the presence of a dispersing agent by acidification. This is then circulated in the goods and dyeing takes place by the gradual addition of caustic soda to reform the soluble and substantive leuco form
Semi-continuous processes of VAT dyes
In semi-continuous vat dyeing processes, the fabric is pigmented by padding with the insoluble vat dye dispersion. Subsequent reduction to the leuco form is a batch operation using a jig or beam dyeing machine. Some dye comes off the fabric during development with the alkaline hydros solution and some vatted dye liquor may be added to the bath in the machine beforehand. This prevents ending in
dyeing. In some cases, an intermediate drying of the padded fabric may be included to minimise colour bleeding into the development bath.
The fully continuous pad-steam process
The pad-steam process for dyeing cellulosic materials with vat dyes is a particularly important process. It involves five stages:
(1) padding the fabric with the fine vat pigment dispersion containing a wetting and an anti-migration agent;
(2) drying of the padded fabric and cooling;
(3) padding of the dried, pigmented fabric with hydros and caustic soda solution;
(4) steaming in air-free steam at about 102-105 °C;
(5) rinsing, oxidation and soaping using a series of wash boxes.
Liquid forms of vat dyes are preferred for continuous dyeing as they give less migration on drying and are more convenient for preparing the large volumes of dye liquor required. The drying of the fabric after padding ensures a high pick-up of alkaline hydros solution from the chemical pad. Some dye invariably bleeds from the fabric into the alkaline hydros solution. This is less when padding a dried
fabric, and an initial addition of some dye to the chemical pad solution minimises colour tailing. Drying of the initially padded fabric is usually carried out in two steps involving a gentle pre-drying stage before the complete drying process. Pre-drying is to control the migration of the pigment particles to the yarn surfaces where water is evaporating. The heating should be as uniform as possible. It is also common practice to use an anti-migrant such as sodium alginate in the first padding solution. The anti-migrant causes flocculation and aggregation of pigment particles during drying thus limiting their movement within the fabric. Some anti-migrant agents may also increase the solution viscosity. The ease of migration depends on the fibres and fabric, the solution pick-up and the pigment particle size
After the chemical pad, the fabric is steamed for 30-60 s in saturated, slightly superheated, air-free steam (Section 12.6.3). It is essential to avoid condensation falling onto the fabric in the steamer as this causes a spotty colour (Section 12.6.3). The steamer has a water seal at its exit with water flowing through the trough to maintain a temperature of not more than 40 °C. After steaming the fabric is rinsed by spraying to remove alkali and hydros. This is followed by rinsing, oxidation, soaping, rinsing and neutralising in a series of wash boxes.
Wet-on-wet processing
The usual pad-steam process cannot be used for corduroy or terry cloth because of the flattening of the pile and excessive migration during drying. A wet-on-wet dyeing process is used. In this, the cloth is padded with the pigment dispersion and then, while still wet, it is padded again with the alkaline hydros solution. The solution pick-up at the chemical pad may go from values of around 70 to 150-200%. The goods then go through the steamer and the usual aftertreatments.
FASTNESS PROPERTIES OF VAT DYES
Many quinone vat dyes provide outstanding colour fastness. The colour gamut is somewhat restricted as there are no bright colours, particularly in the red and turquoise areas. Only azoic and sulphur dyes have smaller colour gamuts. The colour gamut is the size of the 'colour space for the colours obtainable with a particular type of dye either alone or in combined ions.
VAT dyes are very expensive, like reactive dyes. A high degree of overall colour fastness is characteristic of vat dyes. They usually have excellent fastness to washing, chlorine bleaching, alkali boiling a d light. Vat dyes also have the advantage of being the only dyes for cellulosic materials that do not give shade changes on the application of topical reactive finishes for cotton, such as flame-resist finishes (Section 25.3.1). One minor problem is that of photochemical catalytic fading or cotton tendering by a small group of rat dyes. These are mainly yellow and orange vat dyes that photosensitise the tendering of cotton, as in drapery fabrics. The dye functions as a catalyst for the photo-oxidation of the cellulose. Others photosensitise fading of other vat dyes.
DYEING WITH INDIGO AND INDIGOID VAT DYES
Natural Indigo was rapidly displaced from the market once the synthetic product became available. The quality of natural Indigo as quite variable because of the presence of other coloured impurities. The fastne s properties of Indigo dyeings are not up to the standards expected from the vat dy: class as a whole. It is, however, the appearance of faded Indigo in denim that is s.. fashionable today. After dyeing, various wet processes, such as stone washing, deliberately enhance this faded effect. Indigo builds up primarily on the cotton fibre surface. This is one reason for the somewhat inferior fastness properties of Indigo compared to quinone vat dyes.
Application of Indigo to cotton
Dyeing cotton yarn for blue jeans is an important use of Indigo. In a typical batch operation, concentrated reduced Indigo is added to a dye bath from which oxygen has been removed with a little alkaline hydros. The goods are entered and fully immersed to avoid oxidation. After about 15 min at 20-25 °C, the goods are removed and well squeezed before air oxidation. Indigo does not exhaust well because of its limited substantivity for cotton, not surprising considering its small molecular size (Figure 1.4). Deep shades must be built up by repeated dipping in the dye bath after each oxidation. The use of too concentrated a dyebath is not effective for deep shades as it results in poor rubbing fastness. Some salt may be added to aid exhaustion. After dyeing, the goods are well-soaped. The final dyed material may be aftertreated to produce a faded, worn look.
Continuous methods are used for dyeing ball warps, warp beams and piece goods with Indigo. This is usually carried out in a series of 4-6 wash boxes with upper and lower rollers and nips at the exits. The goods are threaded through each box and may be skyed at the mid-point. The first box is used to wet out the material. In subsequent boxes, the goods are immersed in the leuco Indigo solution for 10-30 s at a linear speed of about 25 m min", squeezed and skyed for 2 min to oxidise the leuco dye to Indigo. The boxes are fed with a stock vat of leuco Indigo and the liquor in the boxes is circulated to maintain constant dyeing conditions. This process of several dips and oxidations is then repeated in a second series of boxes, and so on. Several rinsing and washing boxes complete the process.
Indigoid vat dyes
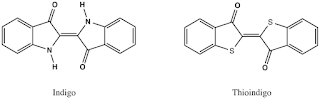
Besides Indigo itself, there are a number of other important indigo and thioindigo derivatives used for dyeing cellulosic fibres. These include chlorinated and brominated indigo and the indigoid dyes in which the nitrogen atoms of Indigo are partially or totally replaced by sulphur atoms (9, in Figure 17.6). Compared to the
anthraquinone type vat dyes and indigoid vat dyes give much paler yellow to brown leuco compound solutions. The vatting process is also slower and requires less alkali.
SOLUBILISED VAT DYES
The solubilised vat dyes provided a means of avoiding the difficult vatting process required for quinone and indigoid vat dyes. The preparation of a solution of a leuco vat dye requires care time, and protection of the solution from excessive exposure to air. The solubilised vat dyes avoid these problems. These dyes are pre-prepared sulphate esters of the leuco vat acid. The first product of this type was derived from Indigo (10, in Figure 17.7). Later solubilised leuco dyes derived from quinone vat dyes were marketed.
 |
| Solubilized Vat Dyes |
Cotton absorbs these dyes directly from a neutral or slightly alkaline solution but they are not very substantive even in the presence of added salt. Although a leuco sulphate ester has the same negative energy as the normal leuco dye, its charge is localised in the sulphate groups wl areas where the phenolate ion is delocalised. Cotton therefore repels a leuco su hate ester molecule more strongly than the normal leuco compound. Because: of their limited substantivity, solubilised vat dyes are generally only suitable for pale shades. After dyeing, the goods are rinsed or hydro-extracted to remove the superficial dye solution, and the var dye pigment is developed in the fibre by oxidation with sodium persulphate or acidified sodium nitrite solution. After neutral sing with dilute soda ash solution, the goods are soaped as in the case of normal v t dyeing. The dyeing has the same fastness properties as one prepared from the original vatted pigment. The low substantivity of the leuco sulphate esters avoids the problems of the high strike of leuco vat dyes. Since they can be used in solutions close to neutrality, they can also be used for wool dyeing. Unfortunately, the dyeing and oxidation conditions for each dye vary so it is important to follow the supplier's instructions. The use of these dyes was never widespread and has declined in recent times. They are not particularly competitive because of their high cost.




Comments
Post a Comment